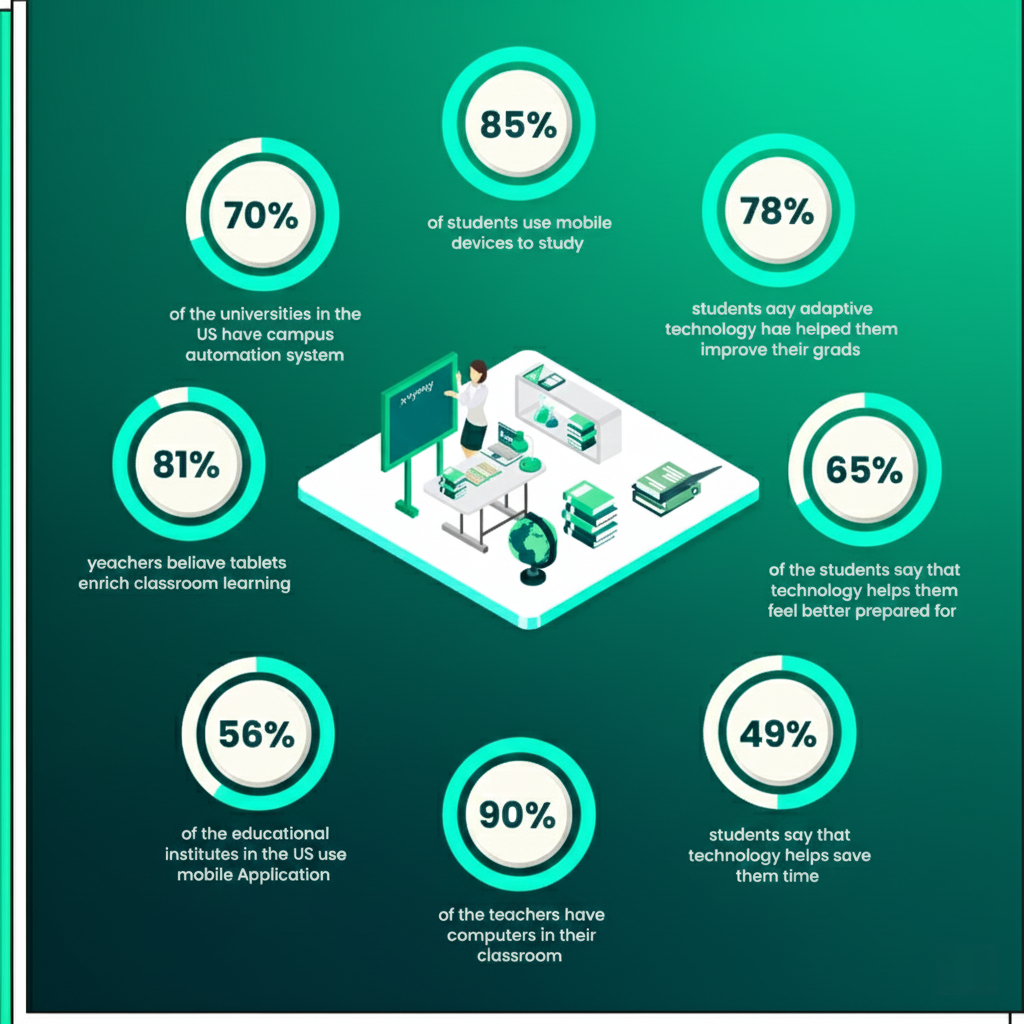
Introduction
Navigating the complex world of US Health Tech regulations is one of the biggest challenges facing today’s CTOs. The rapid pace of technological advancement, increased focus on data privacy, and evolving legislative frameworks have transformed compliance from a “checkbox” exercise into a critical pillar for innovation and growth. In this blog, we’ll break down what every CTO needs to know about simplifying and embracing Health Tech compliance—not just to stay legal, but to accelerate innovation, build market trust, and drive scalable success in the US Health Tech ecosystem.
Understanding the Regulatory Terrain: What CTOs Must Know
HIPAA & HITECH Foundation
HIPAA (Health Insurance Portability and Accountability Act) is the cornerstone of US Health Tech regulation, requiring robust safeguards for Protected Health Information (PHI) including data encryption, access controls, audit logs, and disaster recovery.
HITECH (Health Information Technology for Economic and Clinical Health Act) amplifies HIPAA: penalties for violations are tougher and requirements for breach transparency are stricter.
Emerging Standards & Trends
The FDA alignment with ISO 13485 for device quality management is raising the bar for digital health platforms and connected devices, especially by February 2026.
Interoperability mandates driven by FHIR APIs and Electronic Health Record (EHR) standards mean CTOs must consider not only their own infrastructure but also integrations with external systems and partners.
New regulatory scrutiny around AI in HealthTech, cybersecurity, and diagnostics introduces dedicated compliance frameworks and audit trails for advanced Health Tech products.
Key Considerations for CTOs
-
Compliance isn’t just a legal formality—it must influence technical architecture from the first line of code.
-
Regular risk assessments, comprehensive documentation, and up-to-date staff training are essential for continuous compliance.
Practical Steps to Streamline Compliance and Enable Innovation
Make Compliance a Business Strategy
-
Proactive Planning: Integrate regulatory considerations (HIPAA, HITECH, FDA/ISO, HITRUST) into early product roadmaps instead of post-development fixes.
-
Cross-Functional Teams: Include legal, compliance, and security experts in all major tech decisions, particularly during MVP and scaling phases.
Leverage Modern Compliance Tools
-
Use digital compliance platforms to track regulations, manage documentation, and quickly adapt to new standards—reducing risk and operational costs.
-
Choose cloud vendors and technology stacks with proven compliance certifications (e.g., HITRUST CSF), which streamline audits and boost partner trust.
Continuous Monitoring & Documentation
-
Conduct regular risk audits and vulnerability assessments.
-
Maintain precise documentation of development and user feedback—improving both audit success rates and team accountability.
Balance Innovation and Safety
-
Pilot AI, IoT, and healthcare innovation within legal frameworks, using synthetic data or controlled environments to validate concepts before exposure to real patient data.
-
Ensure any use of third-party APIs and integrations doesn’t introduce unexamined regulatory risks—every touchpoint matters in audits.
Conclusion
CTOs hold the keys to transforming Health Tech compliance from a barrier to an engine for growth and innovation. By architecting compliance into every aspect of product development and leveraging the right processes and tools, leaders can not only meet today’s regulatory requirements but confidently innovate for the future.
Ready to future-proof your Health Tech innovation? Make compliance your launchpad—not your roadblock—grow boldly!
FAQ
Q1: What is the biggest compliance challenge for Health Tech CTOs today?
Staying updated and building scalable systems as HIPAA, HITECH, and FDA/ISO evolve, especially with the rise of AI and interoperability mandates.
Q2: Can compliance slow down Health Tech innovation?
Not if approached proactively; integrating compliance early can actually accelerate product launches and unlock new partnerships by building stakeholder trust.
Q3: How can startups streamline compliance efforts?
Use digital compliance tools, choose compliant vendors, and integrate cross-functional teams from day one for a structured, scalable approach.
Q4: What happens if Health Tech companies fail to comply?
Financial penalties, delayed launches, reputational damage, and even loss of business opportunities—compliance is critical for sustainable growth.
Q5: Are there frameworks that help simplify multi-standard compliance?
Yes, frameworks like HITRUST CSF and automation-led compliance platforms help unify requirements for HIPAA, HITECH, and ISO, making multi-standard compliance more manageable.